Introduction:The spread of SARS-CoV-2 virus continues to pose a major public health threat. Patients with cancer are thought to be at increased risk from SARS-COV-2 infection due to the immunodeficiency that results from the underlying neoplasm and treatment. The immune response to this infection has been the subject of great interest, with an extreme variation in clinical severity between infected individuals. Variation in the immune cell response (B, T, and NK lymphocytes, monocytes, and myeloid derived suppressor cells (MDSCs), among others) and their function have been hypothesized to be responsible for this range of presentation.
Methods:Two patients with a history of hematologic malignancies were matched with three non-cancer patients with similar baseline clinical characteristics and severity of COVID related illness. The critical group (CG) was defined as those requiring mechanical ventilation (MV) due to COVID related respiratory failure and the non-critical group (NCG) were hospitalized but did not require MV. All samples studied were obtained from peripheral blood and processed within 4-hours of collection. Peripheral blood mononuclear cell (PBMC) were isolated using ficoll density gradient separation. Flowcytometric analysis using CytekTM Aurora was done on fresh PBMC samples. Thirty antibody-based flow markers were used to identify 54 distinct immune cell populations. IRB approval was obtained.
Results:
Critical Group (CG):The CG included case 1, a 47 year-old (y.o.) female (F) with a history (hx) of acute myeloid leukemia and had an matched related donor allogeneic hematopoietic stem cell transplant (alloHSCT) 10-years prior remaining in remission, with hematologic recovery, and off immunosuppressants treated with remdesivir and coritcosteroids for COIVD directed therapy; and case 2, a 55 y.o. F with a hx of HIV treated with corticosteroids for COIVD directed therapy (see Figure 1a).
Non-Critical Group (NCG):The NCG included case 3, a 73 y.o male (M) with hx of relapsed/refractory Philadelphia chromosome negative Acute Lymphoblastic Leukemia with loss of CD19 and CD22 expression following treatment with blinatumumab and inotuzumab, and most recently treated with decitabine/venetoclax; case 4, a 66 y.o. M with hx of cardiomyopathy; and case 6, a 54 y.o. M with hx of obesity. None of the NCG cases were treated with COVID directed therapy.
See Table 1 for further clinical information.
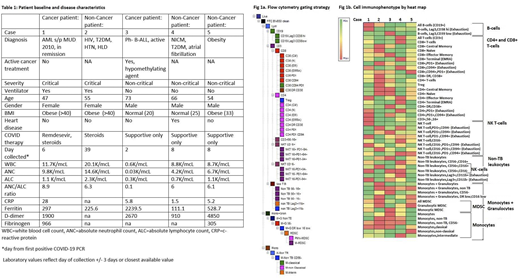
Immunophenotypic expression:Flow cytometry gating strategy done as outlined in Fig 1a. Case 1 had a high proportion of B-cells, CD8+ T-cells, and cells with exhaustion markers (CD8+CD94+ T-cells, CD4+PD1+ T-cells, CD4+PD1+CD94+ T-cells, PD1-CD94+ NK T-cells, Lag3+Cd11b- non-TB leukocytes) and MDSC immunophenotypes compared with matched case 2. Case 3 also had a high proportion of exhaustion markers (Lag3+CD39 low B-cells, CD8+PD1+ T-cells, CD8+CD94+PD1+ T-cells, CD4+PD1+CD94+ T-cells, PD1+CD94+ NK T-cells, PD1-CD94+ NK T-cells, Lag3+CD11b+, Lag3+CD11b- non-TB leukocytes) and high expression of immunosuppressive Treg and all MDSC; although high expression of granulocytic MDSC. Case 2 had a significant number of exhaustion and immunosuppressive cells as well. Cases 4 and 5 had a higher predominance of all T-cell subtypes and also had variable expression of exhaustion and immunosuppressive immunophenotypes (See Fib 1b).
Conclusion:In our study of one critical and one non-critical patient with a history of hematologic malignancy matched with three non-cancer patients we demonstrate the high predominance of exhaustion markers (Lag3,PD1,CD94) and immunosuppressive cell types (Treg, granulocytic and monocytic MDSC). These findings are consistent with the fact that both CG and NCG, as hospitalized patients, represent the most severely ill COVID patient cohort. Of notable interest to the cancer population, cases 1 and 3 had a significant number of exhaustion and immunosuppressive immunophenotypes, suggestive of baseline exhaustion following alloHSCT even years after engraftment in case 1 and attenuated functional immunity in a patient undergoing active treatment in case 3. Interestingly, case 3 had lower expression of all MDSC, a known treatment effect of decitabine. Paired cytokine measurement and its effect on immunophenotype is underway. Additionally, we plan to present an atlas of the peripheral immune cell response on fifteen additional non-cancer COVID patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal